Answer:
Check the explanation
Step-by-step explanation:
When talking about our universe there are 5 d orbitals. The element of first transition series moves away from the universal principles of Hund's rule and Aufbav's principle. So in order to attain stability these elements tend to form half or full filled orbitals.
In our universe the ground state electronic configuration of sixth transition metal, Iron (Fe) : [Ar]
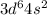
and the electronic configuration of seventh transition metal, Cobalt (Co) : [Ar]
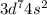
=================================
=================================
In universe L there are seven orbitals.
Ground state electronic configuration of sixth and seven transition element.
Sixth transition metal: [Ar]
![3d^(7) 4s^1 or [X] 3d^(7) 4s^1](https://img.qammunity.org/2021/formulas/chemistry/college/5aye39dgy2dfmuqvryd57pywyxqhh8y2xb.png)
Seventh transition metal: [Ar]
![3d^(7) 4s^(2)or [X] 3d^(7) 4s^(2)](https://img.qammunity.org/2021/formulas/chemistry/college/1jbsawef98wyd83m64awk1gzim9lx39e4x.png)