Answer:
pKa = 3.51
Step-by-step explanation:
The titration of acid solution with NaOH can be illustrated as:

Given that:
Volume of acid solution
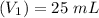
Volume of NaOH
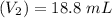
Molarity of acid solution
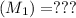
Molarity of NaOH
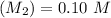
For Neutralization reaction:
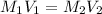
Making
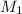 the subject of the formula; we have:
the subject of the formula; we have:
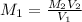
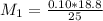
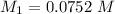
However; since the number of moles of NaA formed is equal to the number of moles of NaOH used : Then :
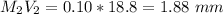
Total Volume after titration = ( 25 + 18.8 ) m
= 43.8 mL
Molarity of salt (NaA ) solution =
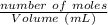
=
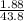
= 0.0429 M
After mixing the two solution ; the volume of half neutralize solution is = 25 mL + 43.8 mL
= 68.8 mL
Molarity of NaA before mixing
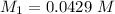
Volume
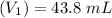
Molarity of NaA after mixing
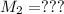
Volume
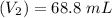
∴
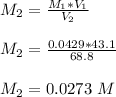
Molarity of acid before mixing = 0.0725 M
Volume = 25 mL
Molarity of acid after mixing =
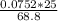
= 0.0273 M
Since this is a buffer solution ; then using Henderson Hasselbalch Equation
![pH = pKa + log ([salt])/([acid])](https://img.qammunity.org/2021/formulas/chemistry/college/xl9nm362zjf5ee8snth0t0ftp19rkr8g70.png)
![3.51= pKa + log ([0.0273])/([0.0273]) \\ \\ 3.51= pKa + log \ 1 \\ \\ 3.51= pKa + 0 \\ \\ pKa = 3.51](https://img.qammunity.org/2021/formulas/chemistry/college/la7ttz4yzi19labl24wid4hc069l31o9sz.png)