Answer:
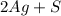 →
→
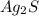
Step-by-step explanation:
In order to balance this, we want to have the same number of elements on each side of the equation.
On the right side, we see that we have two atoms of Ag and one atom of S. So on the left, we want to have 2 atoms of Ag and 1 atom of S. We can put 1 in front of S and 2 in front of Ag:
2Ag + 1S → 1
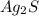
We actually don't have to explicitly write the "1":
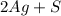 →
→
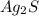
Hope this helps!