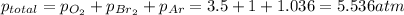Answer:
The total pressure of the tank is 5.536 atm
Step-by-step explanation:
Given:
pO₂ = partial pressure of oxygen = 3.5 atm
pBr₂ = partial pressure of bromine = 760 mmHg = 1 atm
pAr = partial pressure of argon = 105 kPa = 1.036 atm
Question: What is the total pressure inside of the tank in atmospheres, ptotal = ?
First at all, all the pressures must be at the same measure, in this case, atm. The total pressure of the tank is the summation of all partial gas pressures: