Answer:
Option (B) pH > 2.34 and pH < 9.60.
Step-by-step explanation:
In a zwitterionic structure, overall negative charge should be 0.
It means, for glycine, the two functional groups e.g.
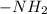 and -COOH should bear equal and opposite charges in it's zwitterionic structure.
and -COOH should bear equal and opposite charges in it's zwitterionic structure.
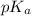 of -COOH (in glycine) = 2.34
of -COOH (in glycine) = 2.34
Therefore, at a pH > 2.34, -COOH group of glycine exists predominantly as
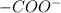 .
.
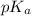 of
of
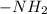 (in glycine) = 9.60
(in glycine) = 9.60
Therefore, at a pH < 9.60,
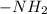 group of glycine exists predominantly as
group of glycine exists predominantly as
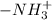 .
.
Hence we can say that at 2.34 < pH < 9.60, glycine exists predominantly in zwitterionic form due to presence of
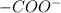 and
and
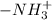 in equal amount.
in equal amount.