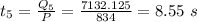Answer:
Step-by-step explanation:
The heat required to change the temperature of steam from 125.5 °C to 100 °C is:
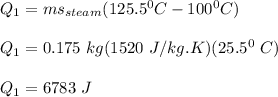
The heat required to change the steam at 100°C to water at 100°C is;

The heat required to change the temperature from 100°C to 0°C is
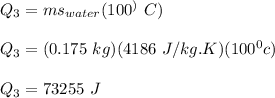
The heat required to change the water at 0°C to ice at 0°C is:

The heat required to change the temperature of ice from 0°Cto -19.5°C is:
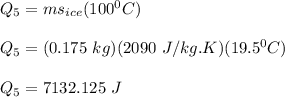
The total heat required to change the steam into ice is:
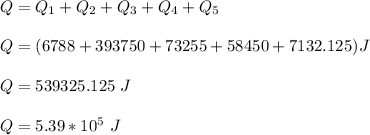
b)
The time taken to convert steam from 125 °C to 100°C is:
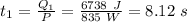
The time taken to convert steam at 100°C to water at 100°C is:
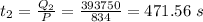
The time taken to convert water to 100° C to 0° C is:
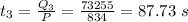
The time taken to convert water at 0° to ice at 0° C is :
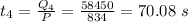
The time taken to convert ice from 0° C to -19.5° C is: