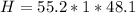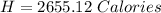Answer:
The number of calories of heat released by the peanut is
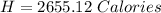
Step-by-step explanation:
Now from the data given we can evaluate the mass of the peanut that is burnt as
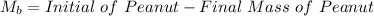
Substituting values
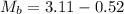
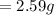
Generally the heat gained by the water can be mathematically represented as

Where
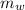 is the mass of water which is given as
is the mass of water which is given as
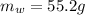
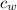 is the specific heat of water which has a constant value of
is the specific heat of water which has a constant value of
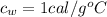
Now is
 is the change in temperature which can be evaluated as follows
is the change in temperature which can be evaluated as follows
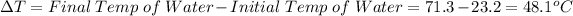
Now