Answer:
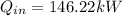
Step-by-step explanation:
Hello,
In this case, the energy balance for the given uninsulated diffuser is:
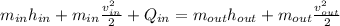
Thus, we shall remember that the inlet mass equals the outlet mass and the outlet velocity is negligible, for that reason we obtain:
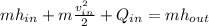
Hence, solving for the heat, we need the enthalpy at the inlet, which at 120°C for a liquid-vapor mixture results:
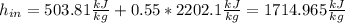
Moreover, at 120 °C the outlet enthlapy as saturated steam is:
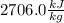
(Values extracted from Cengel, Thermodynamics 7th edition). In such a way, the required heat inlet is:
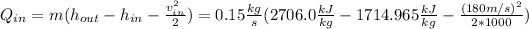
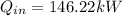
Notice that the term having the velocity should be divided by 1000 to obtain it in kJ/kg.
Best regards.