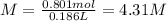Answer:
Molarity = 4.31 M
Step-by-step explanation:
Data:
mass NaOH = 32.02 g
solution volume = 186 mL = 0.186 L
MW NaOH = 39.99 g/mol
The equation for calculating molarity is as follows:
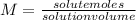
To calculate the moles of NaOH we use the following equation:
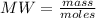
Where MW is the molecular weight of the solute.
We clear the moles of the previous equation:
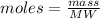
Where the molecular weight of NaOH is 39.99 g/mol.
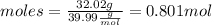
We substitute the values of moles and volume in the molarity equation.