Answer:
The equilibrium concentration of FeSCN²⁺ is 0.0002 M
Step-by-step explanation:
Given data:
9 mL of 0.2 M of Fe(NO)₃
1 mL of 0.002 M of KSCN
Question: What is the equilibrium concentration of FeSCN²⁺
Calculate the initial millimoles of Fe(NO₃)₃:
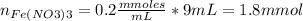
Initial millimoles of KSCN:
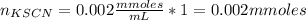
Total volume of solution:
Vtotal = 1 + 9 = 10 mL
Initial concentration of Fe(NO₃)₃:
![[Fe(NO3)3]=(1.8)/(10) =0.18M](https://img.qammunity.org/2021/formulas/chemistry/college/9wgozmj3t97gch7g5k647teg2xuyvled26.png)
Initial concentration of KSCN:
![[KSCN]=(0.002)/(10) =0.0002M](https://img.qammunity.org/2021/formulas/chemistry/college/ggkemg710ue5ucwxep0ju1p5pplbb5el5c.png)
The reaction:
SCN⁻ + Fe³⁺ → FeSCN²⁺
I 0.0002 0.18 0
C -0.0002 -0.0002 0.0002
E 0 0.1798 0.0002
As you see the equilibrium concentration of FeSCN²⁺ is 0.0002 M