Answer : The volume, in milliliters, of the 0.31 M
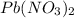 solution that needed is, 50.9 mL
solution that needed is, 50.9 mL
Explanation :
Formula used :

where,
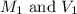 are the initial molarity and volume of dilute
are the initial molarity and volume of dilute
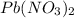 .
.
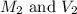 are the final molarity and volume of
are the final molarity and volume of
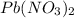 .
.
We are given:
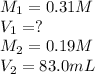
Putting values in above equation, we get:
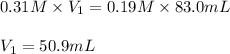
Hence, the volume, in milliliters, of the 0.31 M
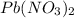 solution that needed is, 50.9 mL
solution that needed is, 50.9 mL