Answer : The correct option is, (D) double replacement reaction
Explanation :
Synthesis reaction : A chemical reaction where multiple substances or reactants combine to form a single product.
It is represented as,
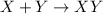
Decomposition reaction : A chemical reaction in which larger reactant decomposes to give two or more than two products.
It is represented as,
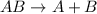
Single replacement reaction : A chemical reaction in which the more reactive element replace the less reactive element.
It is represented as,
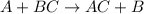
In this reaction, A is more reactive element and B is less reactive element.
Double replacement reaction : It is a type of chemical reaction where a positive cation and a negative anion of two reactants exchange places to form two new products.
It is represented as,
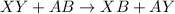
(X and A are the cations, Y and B are the anions)
The given reaction is:

This reaction is a double displacement reaction.