Answer:
6.97 M
Step-by-step explanation:
The reaction that takes place is
H₂(g) + Cl₂(g) ↔ 2HCl (g)
With a equilibrium constant ke =
![([HCl]^2)/([H_(2)][Cl_(2)])](https://img.qammunity.org/2021/formulas/chemistry/college/kixbu5c9cpd2v3lx675udke8m4uwwggw7t.png) = 0.262
= 0.262
The original concentrations for each species are:
250 mL ⇒ 250 / 1000 = 0.25 L
- H₂ = 0.70 mol/0.25 L = 2.8 M
- HCl = 1.6 mol/0.25 L = 6.4 M
The ICE table thus is:
H₂(g) + Cl₂(g) ↔ 2HCl (g)
Initial 2.8 0 6.4
Change -x -x +2x
Equilibium 2.8-x 0-x 6.4 + 2x
- 0.262 =
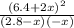
So the equilibrium molarity of HCl is 6.4 + 2*0.285 = 6.97 M