Answer:
(6.668, [infinity])
So then we have this condition:
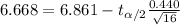
And if we solve for the critical value we got:
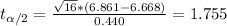
And we can find the probability accumulated in the left of 1.755 with a distribution with degrees of freedom
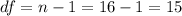 and we got: 0.95, with the following excel code for example:
and we got: 0.95, with the following excel code for example:
"=T.DIST(1.755,15,TRUE)"
So then the confidence level would be 95%
Explanation:
For this case we have the following info:
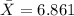 represent the sample mean
represent the sample mean
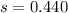 represent the sample deviation
represent the sample deviation
n =16 represent the sample size
For this case the scientists calculate a lower bound confidence interval given by:
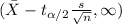
And the interval given is:
(6.668, [infinity])
So then we have this condition:
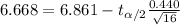
And if we solve for the critical value we got:
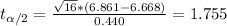
And we can find the probability accumulated in the left of 1.755 with a distribution with degrees of freedom
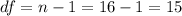 and we got: 0.95, with the following excel code for example:
and we got: 0.95, with the following excel code for example:
"=T.DIST(1.755,15,TRUE)"
So then the confidence level would be 95%