Answer:
+2
Step-by-step explanation:
Oxygen ions always have the charge of -2. Since Fe is in the transition metal stage, it doesn't have a specific set of ions unless specifically shown. In the equation FeO, we would have to have a +2 charge when equalling the two atoms together. So when combines the charges would look like:
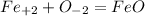
I am happy to help with any other questions you might have, and I will continue to work to providing the best answers for you :)