Answer:
The number of moles of Fe are, 139.9 moles.
The number of moles of CO are, 7.89 moles.
The number of moles of Al are,
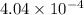 moles.
moles.
Explanation :
As we know that 1 mole of substance contains
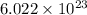 atoms or molecules.
atoms or molecules.
Part 1:
As,
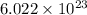 atoms of Fe present in 1 mole
atoms of Fe present in 1 mole
So,
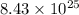 atoms of Fe present in
atoms of Fe present in
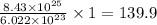 mole
mole
Thus, the number of moles of Fe are, 139.9 moles.
Part 2:
As,
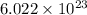 molecules of CO present in 1 mole
molecules of CO present in 1 mole
So,
 molecules of CO present in
molecules of CO present in
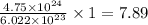 mole
mole
Thus, the number of moles of CO are, 7.89 moles.
Part 3:
As,
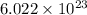 atoms of Al present in 1 mole
atoms of Al present in 1 mole
So,
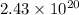 atoms of Al present in
atoms of Al present in
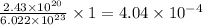 mole
mole
Thus, the number of moles of Al are,
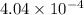 moles.
moles.