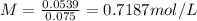Answer:
The molarity of the solution is 0.7187 mol/L
Step-by-step explanation:
Given data:
m = mass of NaCl = 3.15 g
V = volume = 75 mL = 0.075 L
Question: What is molarity of the solution, M = ?
The molarity is the number of moles of the compound divided by volumen. In this case, it is necessary calculate the number of moles. The molecular weight of NaCl is 58.44 g/mol.
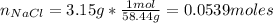
The molarity: