Answer : The correct option is, (d) +1.201 V
Explanation :
Here cadmium (Cd) undergoes oxidation by loss of electrons, thus act as anode because less value of electrode potential. Silver (Ag) undergoes reduction by gain of electrons and thus act as cathode because more value of electrode potential.
The half-cell reactions are:
Oxidation reaction (anode) :
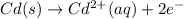
Reduction reaction (cathode) :
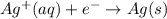
The balanced cell reaction will be,
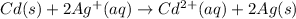
Given :
![E^o_([Cd^(2+)/Cd])=-0.402V](https://img.qammunity.org/2021/formulas/chemistry/high-school/gp0j4oapwo4vbu1vdubjp17zdfvf04z1v3.png)
![E^o_([Ag^+/Ag])=+0.799V](https://img.qammunity.org/2021/formulas/chemistry/high-school/buo3maeh0acjlynkv1dfarab3peurm6xn9.png)
![E^o=E^o_([cathode])-E^o_([anode])](https://img.qammunity.org/2021/formulas/chemistry/high-school/zv7mmv2m9h5mm56psgihgdmavjqsgde18d.png)
![E^o=E^o_([Ag^+/Ag])-E^o_([Cd^(2+)/Cd])](https://img.qammunity.org/2021/formulas/chemistry/high-school/52gakkkstm76jo7wl96dlh5igqu7lk8ccu.png)
Now put all the values in this expression, we get:
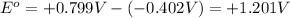
Therefore, the standard cell voltage is, +1.201 V