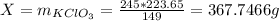Answer:
367.7466 g of potassium chlorate is produced.
Step-by-step explanation:
Given data:
223.65 g of potassium chloride is produced
Question: How much potassium chlorate did the reaction start with, mKClO₃ = ?
The balanced reaction:
2KClO₃ → 2KCl + 3O₂
Information needed:
Molecular weight of KClO₃ = 122.5 g/mol
Molecular weight of KCl = 74.5 g/mol
Molecular weight of O₂ = 32 g/mol
You can see in the reaction that 2 moles of KClO₃ gives 2 moles of KCl.
Mass of KClO₃:

Mass of KCl:
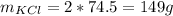
245 g KClO₃ --------- 149 g KCl
X g KClO₃ ------------- 223.65 g KCl