Answer:
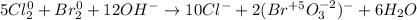
Step-by-step explanation:
Hello,
In this case, for the given reaction, we first identify each element's oxidation state:
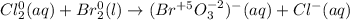
Hence, it seen that chlorine is reduced whereas bromine is oxidized, thereby, the half-reactions in acidic media (first step) are:
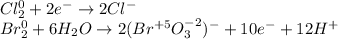
Then we balance the electrons:
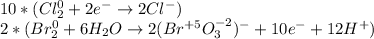
_______________________________________
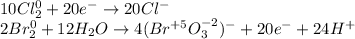
Finally, we add as many OH⁻ as many H⁺ we have in order the obtain water and OH⁻ at each side once the half reactions are added:
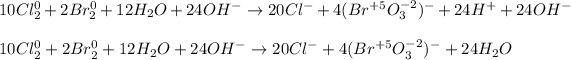
So the simplified result is:
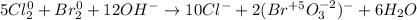
Best regards.