Answer:
Final pressure will be equal to 394.94 kPa
Step-by-step explanation:
It is given volume
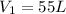
Temperature of the gas
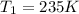
Initial pressure exerted
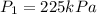
Now volume is decreased to
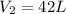
And temperature is increases to
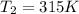
By using gas equation
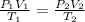
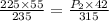
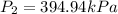
So final pressure will be equal to 394.94 kPa