Answer:
The pressure exerted by helium on the walls of the container is approximately 760.6 mmHg
Step-by-step explanation:
The parameters given in the question are;
Amount of moles of helium in balloon = 1.00 mole
Volume of balloon = 22.4 L = 0.0224 m³
Temperature of balloon = 0.00°C = 273.15 K
Therefore, we have the pressure given by the universal gas equation as follows;
P·V = n·R·T
So that
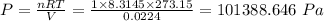
Converting the resultant pressure in pascals, Pa to millimetre mercury, mmHg we have;
1 mmHg = 133.3 Pa
∴ 1 Pa = 1/133.33 mmHg and
101388.646 Pa = 101388.646× 1/133.3 mmHg = 760.604996 mmHg
Therefore the pressure exerted by helium on the container ≈ 760.6 mmHg.