Answer:
28g remain after 13.5 hours
Step-by-step explanation:
Element decayment follows first order kinetics law:
ln[Pa-234] = -kt + ln [Pa-234]₀ (1)
Where [Pa-234] is concentration after t time, k is rate constant in time, and [Pa-234]₀ is initial concentration
Half-life formula is:
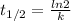
6.75 = ln2 / k
k = 0.1027hours⁻¹
Using rate constant in (1):
ln[Pa-234] = -0.1027hours⁻¹×13.5hours + ln [112.0g]
ln[Pa-234] = 3.332
[Pa-234] = 28g after 13.5 hours