Answer:
B
Explanation:
Notice that the equation shows
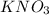 going from a solid to an aqueous solution, which is dissolved. Entropy is essentially when a system becomes more disordered. Whenever we go from solid to liquid to gas, that's becoming more disordered because the molecules in the system are moving much more and in more random directions.
going from a solid to an aqueous solution, which is dissolved. Entropy is essentially when a system becomes more disordered. Whenever we go from solid to liquid to gas, that's becoming more disordered because the molecules in the system are moving much more and in more random directions.
In addition, when we dissolve solids into water, that's another increase in entropy because we technically have a liquid component here and the molecules are now breaking apart.
So, the entropy here will increase.
Hope this helps!