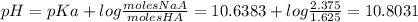Answer:
The new pH is 10.8031
Step-by-step explanation:
Given information:
[NaA] = concentration = 2 M
[HA] = concentration = 2 M
V = volume = 1 L
15 g of NaOH added
Question: What is the new pH, pH = ?
Moles of NaA:
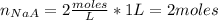
Moles of HA:
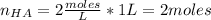
Moles of NaOH:
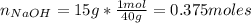
The reaction:
HA + NaOH → NaA + H₂O
Moles of NaA = 2 + 0.375 = 2.375 moles
Moles of HA = 2 - 0.375 = 1.625 moles
The value of Ka is 2.3x10⁻¹¹
The pKa:
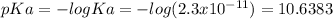
Applying the Henderson's Hasselbach equation