Answer:
The number of moles of nitrogen gas in the tennis ball is 0.0124 moles
Step-by-step explanation:
Here we have the universal gas equation as
P·V = n·R·T
Where:
P = Pressure of the gas = 212 kPa
V = Volume of the gas = 0.148 L = 0.000148 m³
n = Number of moles
R = Universal gas constant = 8.3145 J / mol·K.
T = Temperature of the gas in Kelvin = 32 + 273.15 = 305.15 K
Therefore,
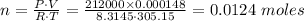
The number of moles of nitrogen gas in the tennis ball = 0.0124 moles.