Answer:
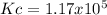
Step-by-step explanation:
Hello,
In this case, we are talking about the following reaction at equilibrium:
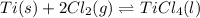
Which has the following associated law of mass action:
![Kc=(1)/([Cl_2]^2)](https://img.qammunity.org/2021/formulas/chemistry/college/c1e1psf514xv4vj675fdiygttz2epxk5yj.png)
As just the gases or the aqueous solutions are said to have concentrations, that is why just chlorine participates in that equation. For that reason, we compute the concentration of chlorine at equilibrium in terms of its molarity:
![[Cl_2]_(eq)=(1.08gCl_2*(1molCl_2)/(70.9gCl_2))/(5.2L)=2.93x10^(-3)M](https://img.qammunity.org/2021/formulas/chemistry/college/yfmeu26x42y274g9dm3lmtzfmolpv2zs41.png)
Finally, the equilibrium constant results:
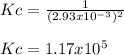
Best regards.