Answer : The final number of moles of gas that withdrawn from the tank to lower the pressure of the gas must be, 0.301 mol.
Explanation :
As we know that:
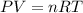
At constant volume and temperature of gas, the pressure will be directly proportional to the number of moles of gas.
The relation between pressure and number of moles of gas will be:
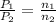
where,
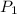 = initial pressure of gas = 24.5 atm
= initial pressure of gas = 24.5 atm
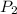 = final pressure of gas = 5.30 atm
= final pressure of gas = 5.30 atm
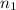 = initial number of moles of gas = 1.40 moles
= initial number of moles of gas = 1.40 moles
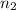 = final number of moles of gas = ?
= final number of moles of gas = ?
Now put all the given values in the above expression, we get:
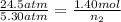
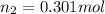
Therefore, the final number of moles of gas that withdrawn from the tank to lower the pressure of the gas must be, 0.301 mol.