Answer:
Chloroform is expected to boil at 333 K (60
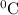 ).
).
Step-by-step explanation:
For liquid-vapor equilibrium at 1 atm,
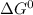 = 0.
= 0.
We know,
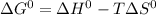 , where T is temperature in kelvin scale.
, where T is temperature in kelvin scale.
Here both
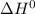 and
and
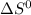 are corresponding to vaporization process therefore T represents boiling point of chloroform.
are corresponding to vaporization process therefore T represents boiling point of chloroform.
So,
![0=(31.4* 10^(3)(J)/(mol))-[T* (94.2(J)/(mol.K))]](https://img.qammunity.org/2021/formulas/chemistry/college/a4i94jvtkuh7ns7ie15nuqcw77viq6ydoi.png)
or, T = 333 K
So, at 333 K (60
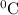 ) , chloroform is expected to boil.
) , chloroform is expected to boil.