Answer:
The mass of helium added is approximately 0.7 grams
Step-by-step explanation:
Here we have
Initial mass of helium gas in the cylinder = 2.00 g
Initial pressure of gas = Final pressure of gas
Initial volume of gas = 2.00 L
Final volume of gas = 2.70 L = 0.0027 m
Initial temperature of the gas = room temperature = 21 °C
Molar mass of helium gas = 4.003 g/mol
Therefore, the number of moles, n of helium is given as
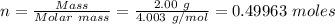
The pressure of the gas in the cylinder is given by;
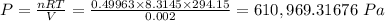
Therefore, when the volume is increased to 2.7 by adding more helium, we have
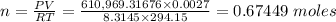
That is the number of moles of helium added is given by;
0.67449 - 0.49963 = 0.174864 moles
Mass of helium added = Number of moles added × Molar mass of helium
0.174864 moles × 4.003 g/mol = 0.69998111 ≈ 0.7 grams.