Answer:
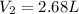
Step-by-step explanation:
Hello,
In this case, we use the Charles' law which allows us to understand the volume-temperature behavior as gas has as a directly proportional relationship:
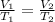
Whereas the temperature must be in absolute units such as Kelvin. Hence, solving for the volume at 43.0 °C, we obtain:
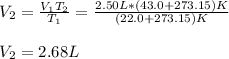
Best regards.