Question:
Options
A) increases, increases, increases
B) increases, increases, decreases
C) increases, decreases, increases
D) increases, decreases, decreases
E) none of the above
Answer:
The correct option is;
A) increases, increases, increases
Step-by-step explanation:
Here we have the equations of the kinetic theory of gases as follows
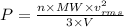 .........(1)
.........(1)
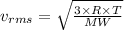 .........(2)
.........(2)
Where
P = Pressure
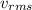 = Root mean square velocity
= Root mean square velocity
MW = Molecular weight
V = Volume
R = Universal gas constant
T = Temperature
From the above it is seen that when the temperature of the gas is increased the average velocity increases
Also at constant pressure, according to Charles law an increase in temperature will lead to an increase in volume volume
While according to Avogadro's law at constant pressure and temperature, the force of molecule to wall collision is constant increasing the temperature increases the average force and frequency of individual collisions, more so according to equation (2).