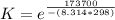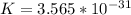Answer:
The equilibrium constant is
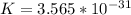
Step-by-step explanation:
From the question we are told that
The reduction potential of
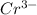 =
=
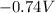
The reduction potential of
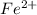 =
=
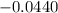
In order for us to obtain the equilibrium constant we need to understand the process of the reaction
At the cathode
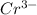 is reduced to Cr the reaction is
is reduced to Cr the reaction is
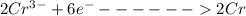
At the anode
 is oxidized to
is oxidized to
 the reaction is
the reaction is
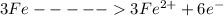
The net voltage of the cell is mathematically given as
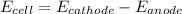
substituting value
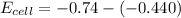
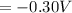
Next we need to mathematically evaluate the free energy of the cell as
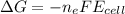
where
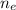 is the number of moles of electron which is 6
is the number of moles of electron which is 6
F is the farad constant with a value
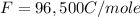
Substituting values
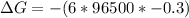
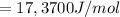
The equilibrium constant is mathematically represented as
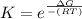
Where R is the gas constant with value 8.314 J/mol
T is the temperature with a given value of
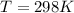
Substituting values