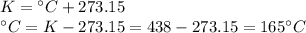Answer:
165 °C
Step-by-step explanation:
Given data
- Initial pressure (P₁): 660 kPa
- Initial temperature (T₁): 602 °C
- Final pressure (P₂): 330 kPa
- Final temperature (T₂): ?
Step 1: Convert the initial temperature to Kelvin
When working with gases, we always have to use the Kelvin scale. We can convert from Celsius to Kelvin using the following expression.
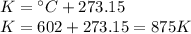
Step 2: Calculate the final temperature
We will use the Gay-Lussac's law.
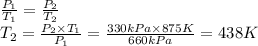
Step 3: Convert the final temperature to Celsius