50 litres of magnesium chlorate solution can be made by diluting 250 ml of 10 M HCl.
The correct option is 2.
Step-by-step explanation:
Data given:
Molarity of magnesium chlorate M1 = 0.05 M
volume of the magnesium chlorate V1 = ?
Molarity of the HCl solution = 10 M
volume of the HCl solution = 250 ml or 0.25 litres
the formula used is for dilution
M1V1 = M2V2
rearranging the equation to get the volume of magnesium chlorate solution,
V1 =
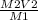
putting the values in the equation:
V1 =
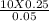
V1 = 50 LITRES
thus the 0.05 M of the magnesium chlorate solution can be made by diluting the given solution with 50 litres.