4.2 X
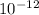 moles/litre is the hydroxide ion concentration of the solution if hydrogen-ion concentration of 2.3x
moles/litre is the hydroxide ion concentration of the solution if hydrogen-ion concentration of 2.3x
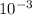 .
.
Step-by-step explanation:
Data given:
hydrogen ion concentration = 2.3 X
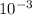
Hydroxide ion concentration =?
first the pH of the solution is calculated by using the formula:
pH = - log[
 ]
]
Putting the values in the equation,
pH = -
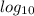 [2.3 X
[2.3 X
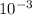 ]
]
pH = 2.63
Now from the relation we will find the pOH of the solution:
14 = pOH + pH
14 - pH = pOH
14 - 2.63 = pOH
11.37 = pOH
now using the formula to calculate OH ions in the solution as:
pOH = -
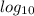 [
[
 ]
]
[
 ] =
] =
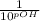
= 4.2 X
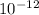 moles/litre is the hydroxide ion concentration of the solution.
moles/litre is the hydroxide ion concentration of the solution.