108.05 grams of NH4NO3 would you need to decompose to inflate your air bag to the correct size with 59.6g of dinitrogen monoxide.
Step-by-step explanation:
Data given:
mass of dinitrogen monoxide = 59.6 grams
atomic mass of dinitrogen monoxide = 44.01 grams/mole
grams of N
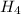 N
N
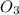 = ?
= ?
atomic mass of ammonium nitrate = 108.05 grams/mole
balanced chemical equation:
N
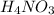 ⇒
⇒
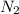 O +2
O +2
 O
O
Firstly the number of moles will be calculated from the given mass by using the formula:
number of moles =

putting the values in the equation:
number of moles =
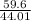
number of moles = 1.35 moles
from the balanced equation :
1 mole of ammonium nitrate decomposes to release 1 mole of dinitrogen monoxide
so, 1.35 moles will give 1.35 moles of dinitrogen monoxide.
mass = atomic mass x number of moles
mass = 80.043 x 1.35
mass = 108.05 grams of ammonium nitrate.