The new temperature is 337.21 K when the pressure was reduced to 101.3 kPa from 150.2 kPa at 500 K.
Step-by-step explanation:
Data given:
volume of the container = 100 ml
initial pressure on the container P1 = 150.2 kPa
Initial temperature of the container, T1 = 500 K
final temperature of the container, T2 = ?
Final pressure on the container P2 = 101.3 kPa
from the data provided, we will use Gay Lussac's law to calculate the final temperature:
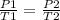
rearranging the equation,
T2 =
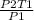
Putting the values in the equation:
T2 =
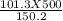
T2 = 337.21 K
thus, the final temperature of the container is 337.21 K