0.044 M is the molarity of 1.60 L of a solution containing 8.53 g of dissolved KBr.
Step-by-step explanation:
Data given:
volume of the solution = 1.60 litres
mass of KBr = 8.53 grams
atomic mass of KBr = 119.002 gram/moles (K = 39.09 g/mole + 79.9 g/mole)
number of moles =?
molarity of KBr solution =?
First the number of moles will be calculated by using the formula:
number of moles =

putting the values in the equation:
number of moles =
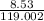
number of moles = 0.071 moles of KBr
now molarity is calculated using the formula:
molarity =
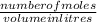
putting the values in the equation:
molarity =
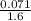
= 0.044 M
molarity of KBr solution is 0.044 M