The formation of white precipitate of calcium carbonate indicates that a chemical change has occurred in the reaction.
Step-by-step explanation:
A chemical change is the one in which reactants react to produce a new product or substance. The reaction is mostly not reversible.
In case of aqueous calcium hydroxide treated with carbon dioxide gas produces chalk which is in the form of precipitate. This chalk is a different chemical compound from the reactants. Calcium carbonate is the chemical formed as precipitate.
The chemical reaction is shown as:
Ca(
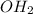 +
+
 ⇒ CaC
⇒ CaC
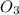 ↓ + water
↓ + water
downward arrow indicates precipitation.
Thus the formation of a new compound as precipitate shows that a chemical change has occurred in the reaction.