Answer:
The average kinetic energy of the molecules increases
Step-by-step explanation:
The temperature of a substance is proportional to the average kinetic energy of the particles in the substance.
In fact, for an ideal gas for instance, there is the following relationship:
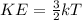
where
KE is the average kinetic energy of the particles
k is the Boltzmann's constant
T is the absolute temperature of the gas
When we heat a substance (such as the flask of water in this problem), we are giving thermal energy to the particles of the substance; therefore, these particles will move faster on average, so their kinetic energy will increase (and the temperature of the substance will increase as well).