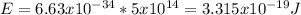Answer:
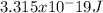
Step-by-step explanation:
Using Eisten's formula, energy is a product of Plank's constant and photon frequency
E=hf
Where f represent frequency, h is Plank's constant and E is energy.
Substituting frequency with 5 x 10^14 Hz and
Planck's constant is 6.63 x 10^-34 J•s.