Answer:
Explanation:
Solution:-
- The conclusions of Bohr's study have gave us hints regarding the probability of finding an electron ( e- ) in a 3 dimensional space of a nucleus.
- In Bohr's model the the 3-dimensional was considered as a spherical shell with thickness ( t = δr ) . Where ( r ) is the absolute radius of the density of electrons ( e - ) found in the vicinity of a nucleus.
- Bohr performed several experiments to determine what is the probability of finding of finding a single electron ( e- ) in a atom around its nucleus.
- He found that the probability P of finding an electron is a function of radial distance ( r )^2 - square of its distance from nucleus and the atom's wave-function R ( r ). The probability of the distribution is given as:
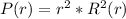
- Where R ( r ) is the wave-function specific for an atom. Here we will investigate an Hydrogen atom which has an orbital configuration = 1s orbitals.
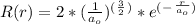
Where,
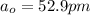 , is the Bohr's radius.
, is the Bohr's radius.
- We will determine the probability P ( r ) of finding that electron in a hydrogen atom at a radial distance r = 1.1a_o.
- Determine the P ( R ) by performing an integral from the center of spherical shell i.e nucleus r = 0 to r = 1.1a_o:
![P ( r ) = \int [ 2*((1)/(a_o) )^(^(3)/(2)^) * e^(^-^(r)/(a_o)^) ] ^2*r^2 dr\\\\P ( r ) = \int [ 4*((1)/(a_o) )^(^3^) * e^(^-^(2r)/(a_o)^) ] *r^2 . dr\\\\P ( r ) = 4*((1)/(a_o) )^(^3^) \int [ e^(^-^(2r)/(a_o)^) . r^2 ] . dr](https://img.qammunity.org/2021/formulas/mathematics/college/pnfsolb0ya8k6r4d64jusog1rsdlgcpqqt.png)
- Perform integration by parts:
![P ( r ) = 4*((1)/(a_o) )^(^3^) * ( [ (e^(^-^(2r)/(a_o)^) )/((-2)/(a_o) ) ]*r^2 - \int [ e^(^-^(2r)/(a_o)^) . 2r ] . dr)](https://img.qammunity.org/2021/formulas/mathematics/college/2guzvlrr5yp2mds26bvvmc4j2uytds0cnr.png)