Answer:
The age of the Igneous Sill is 1.456 × 10¹⁰ years
Step-by-step explanation:
The radioactive decay is given as follows;
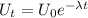
Also, where 1 Uranium 238 atom disintegrates to produce 1 Lead 206 atom, we write;
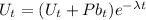
Such that;
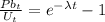
The decay constant, λ is given by the following;
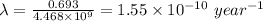
Percentage by mass of Uranium = 91.7%
Percentage by mass of Uranium = 8.3%
Number of moles of Uranium 238 and Lead 206 present is therefore;
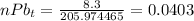
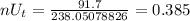
Therefore we have;
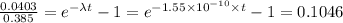
From which t is found to be t = 14555074285.2 = 1.456 × 10¹⁰ years.