Answer:
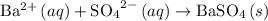 .
.
Step-by-step explanation:
Make use of the fact that calcium sulfate
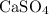 and magnesium sulfate
and magnesium sulfate
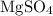 are much more soluble in water than barium sulfate
are much more soluble in water than barium sulfate
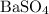 .
.
When sulfate ions
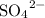 are added to dilute solutions containing
are added to dilute solutions containing
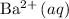 ,
,
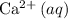 , and
, and
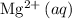 , precipitation would be visible only in the solution with
, precipitation would be visible only in the solution with
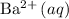 . Barium sulfate would be the precipitate.
. Barium sulfate would be the precipitate.
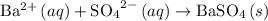 .
.
This ionic equation is balanced as it conserves both the atoms and the charges on the ions.