The coefficients of the hydrocarbon and water is 61 and it is explained below.
Step-by-step explanation:
We have to balance the chemical equation that describes the combustion of octane C₈H₁₈. The combustion of octane involves burning this hydrocarbon in the presence of excess oxygen,O 2. Because octane is a hydrocarbon, that is the compound that contains only carbon and hydrogen, the reaction will produce two products carbon dioxide CO 2 and water H 2 O.
The unbalanced chemical equation is
C₈H₁₈ + O₂ → CO₂ + H₂O
To balance this equation, we have 8 present on the reactant side, so multiply the carbon dioxide by 8 to get 8 atoms of carbon on the products side.
C₈H₁₈ + O₂ → 8 CO₂ + H₂O
Now, we have 18 on the reactant side and 2 on the products side, so multiply the water molecule by 9 to get
C₈H₁₈ + O₂ → 8 CO₂ + 9 H₂O
8 * 2 atoms O + 9 *1 atoms O = 25 atoms O
By adding fractional coefficient to O₂ we get
C₈H₁₈ +
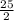 O₂ → 8 CO₂ + 9 H₂O
O₂ → 8 CO₂ + 9 H₂O
Multiply all the coefficients by 2 we get,
2C₈H₁₈ + (
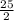 * 2) O₂ → 8 * 2 CO₂ + 9* 2 H₂O
* 2) O₂ → 8 * 2 CO₂ + 9* 2 H₂O
The balance equation is
2C₈H₁₈ + 25 O₂ → 16 CO₂ + 18 H₂O
Add the coefficients to get their sum
= 2 +25 + 16 +18 = 61
Thus, The coefficients of the hydrocarbon and water is 61