There are 8.4 × 10¹⁰ atoms in the given sample of Nickel.
Step-by-step explanation:
To find the number of atoms in the given mass of Nickel, first we have to find the number of moles using its mass and molar mass of nickel then multiply the moles by the Avogadro's number so that we will get the number of atoms in that sample of nickel.
Moles of Nickel =
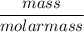
=
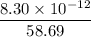
= 1.4 × 10⁻¹³ moles
Now we have to multiply the number of moles by Avogadro's number, we will get the number of atoms of Nickel.
Number of atoms = 1.4 × 10⁻¹³× 6.022 × 10²³
= 8.4 × 10¹⁰ atoms of Nickel