The final temperature of the Argon gas is 313.5 K.
Step-by-step explanation:
Using the ideal gas equation, we can find the final temperature of the Argon gas.
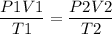
Now we have to rearrange the equation to get T2 as,
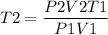
P1 = Initial pressure = 720 mm Hg = 1 atm
P2 = Final Pressure = 360 mm Hg = 0.5 atm
V1 = initial volume = 1 L
V2 = Final Volume = 2.14 L
T1 = Initial Temperature = 293 K
T2 = Final Temperature = ?
Now we have to plugin the values as,
T2 =
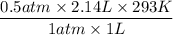
= 313.5 K
So the final temperature of the Argon gas is 313.5 K.