The enthalpy change for the reaction is -296.8 kJ.
Step-by-step explanation:
S(s) + O₂(g) ⇄ SO₂ (g)
We have to find the enthalpy change by finding the difference between the sum of enthalpies of products and that of reactants along with their coefficient terms. It is termed as ΔH.
It is found that,
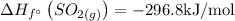
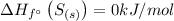
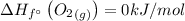
The enthalpy change can be found out as,
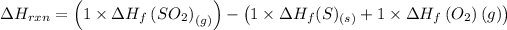
= (1 × -296.8) - ((1 × 0) + ( 1 × 0 ))
= -296.8 kJ
So the enthalpy change for the reaction is -296.8 kJ/ mol.