Answer:
As pressure increases, the volume of an object tends to decrease.
Step-by-step explanation:
As pressure increases, the volume of an object tends to decrease.
Since it is described by Boyle's Law, which states that the pressure and volume of a gas are inversely proportional when the temperature and the amount of gas are held constant.
The law was first published by the Irish physicist Robert Boyle in 1662. Boyle's law can be expressed mathematically as follows:
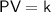
where P is the pressure of the gas, V is the volume of the gas, and k is a constant.

For example,
- Scuba diving:
As the diver descends, water pressure increases, so the volume of air in the lungs decreases.
The diver needs to breathe out to equalize the pressure. - Air conditioning:
Air conditioners compress air, increasing its pressure.
When the compressed air is released, its volume decreases, which cools the air.